Plant systems for bioproduction
Workpackage J: Virus-based systems for bioproduction
The CPMV-HT (Cowpea Mosaic Virus-HyperTranslatable) expression system, developed by Prof George Lomonossoff and Dr Frank Sainsbury at the John Innes Centre, has established a unique position for the UK for rapid transient expression of proteins in plants, through Agrobacterium-mediated infiltration of Nicotiana benthamiana leaves. The technology is powerful and was used under licence by the Canadian company Medicago to produce 10M effective doses of H1N1 (swine flu) VLP Vaccine in just 30 days, while the traditional route would have taken 9 – 12 months. Taking a synthetic biology approach, researchers at the OpenPlant are finding the CPMV-HT technology highly amenable for testing prototype plant natural product biosynthetic pathways, and for the actual production of high-value chemicals and bioactive proteins. The CPMV-HT is a highly flexible system and is being adopted for a range of applications in the field of plant synthetic biology.
The process of agroinfiltration. (Image credit: George Lomonossoff and Eva Thuenemann (JIC), from Norwich Research Park Image Library, NRP-134)
Modular DNA cassettes for transient expression
OpenPlant researchers are creating standardised and modular viral expression cassettes with fine-tuned levels of translation efficiency, using the CPMV-HT (Hypertrans) system. Some of the recent work is published in Peyret et al., (2019). Further, the Hypertrans system is being modified to permit expression in alternative hosts, including the development of new bacterial strains for the intra-cellular delivery of viral vectors to a range of new plant hosts. The Hypertrans system has been used successfully in the BY2 cell pack system by the Lomonossoff group, is being used in tomatoes by the Martin lab, and is being tested in Marchantia in collaboration with the Haseloff lab. The Hypertrans plant expression system has proved to be highly amenable for expression of plant natural product biosynthetic pathways, and has been integrated into the Osbourn lab’s synthetic biology pipeline. A recent publication in the Journal of Visual Experimentation (Stephenson et al., 2018) provides a video introduction to the full pipeline and the accompanying methods. The Dupree lab are also utilising the Hypertrans system to express different glycosyltransferases for use in in vitro assays. Research on the use of the Hypertrans system for the expression of proteins in micro-algae is currently underway in collaboration with Sogang University, S. Korea
Leaf Systems, Norwich Research Park
Production facility
In a separate development, Leaf Systems® International Limited has opened a facility on the Norwich Research Park that provides a production facility for the expression and production of proteins, metabolites and complex natural products for research and bio-medical applications using the proprietary Hypertrans® expression technologies developed by Prof. George Lomonossoff and colleagues. The Hypertrans system allows for the simultaneous production of multiple gene products in a controlled and coordinated manner within the tissues of plants. Using gene synthesis and modular vectors, new products can be developed, validated, scaled and produced very quickly.
Applications of the technology include:
Production of complex proteins, such as antibodies or enzymes for research applications.
Production of antigens including virus-like particles for vaccine development or nano-technology.
Rapid prototyping and surge production in response to external circumstances.
Metabolic pathway engineering to generate complex biochemicals that are difficult or impossible to synthesise chemically or are only found in rare species or trace amounts in nature.
Leaf Systems is employing state of the art containment facilities to produce and engineer its feedstock plants and has industry standard downstream processing capabilities to ensure production quality and bio-security.
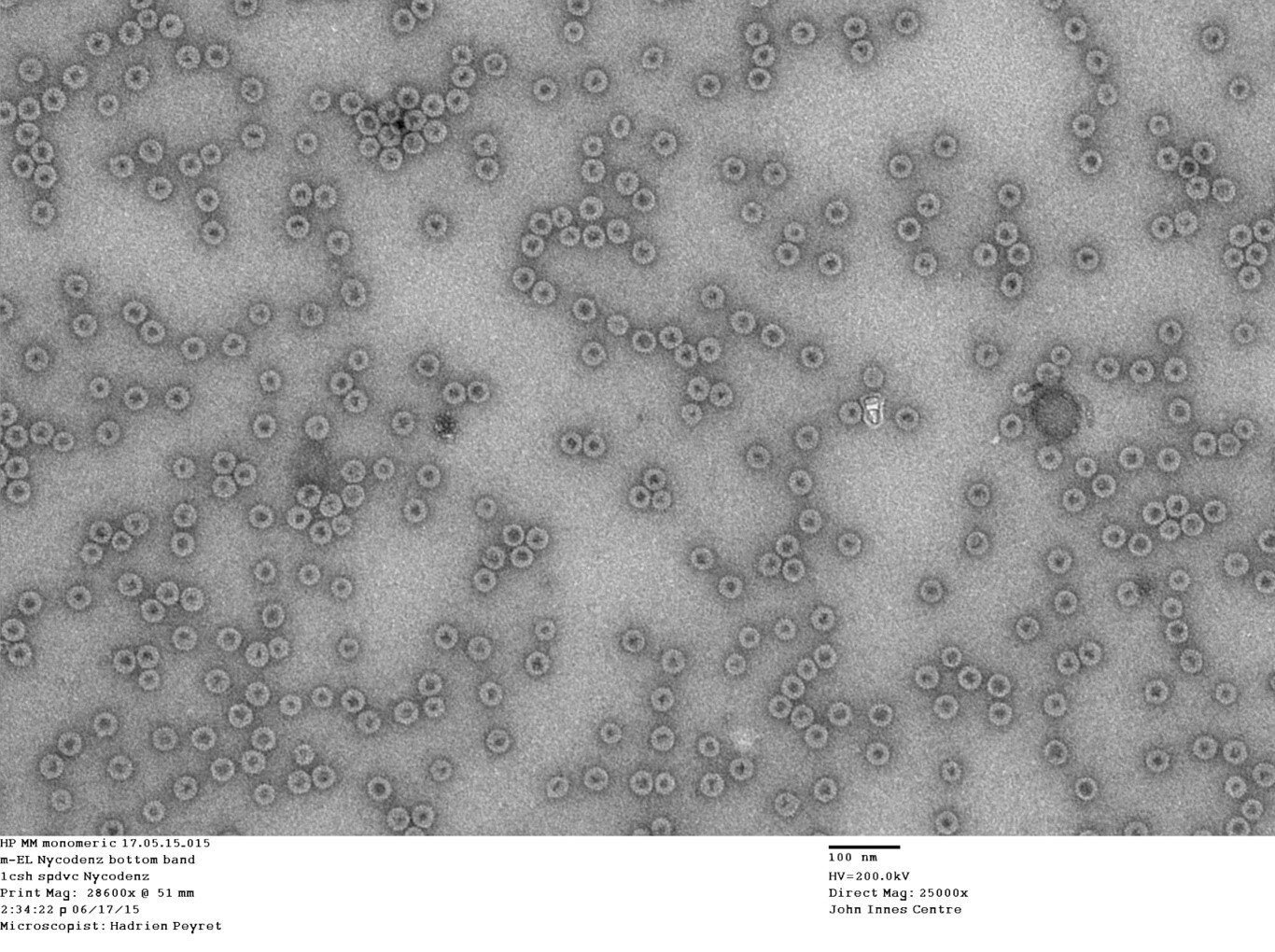
Virus-like particles produced in plants. Image credit: Hadrien Peyret, John Innes Centre. NRP image library NRP-169.
Production of synthetic poliovirus particles in plants
As part of a World Health Organisation (WHO) – funded collaboration involving JIC, University of Leeds, University of Oxford and the National Institute of Biological Standards and Control (NIBSC), the Lomonossoff lab has used the Hypertrans® system to produce synthetic non-infectious poliovirus particles for use in the WHO worldwide polio eradication campaign. The synthetic particles consist only of the protein shell and lack the virus nucleic acid; however, to ensure they are stable enough to act as an effective vaccine, it was necessary to first identify mutations in the protein which stabilised the particles. The stabilised proteins were able to form particles in plants that have a structure equivalent to that of natural poliovirus. These particles were able to protect mice against poliovirus and thus have the potential to act as an effective vaccine.
Publications
Thuenemann EC, Le DHT, Lomonossoff GP, Steinmetz NF (2021) Bluetongue Virus Particles as Nanoreactors for Enzyme Delivery and Cancer Therapy. Mol. Pharmaceutics 18, 3, 1150–1156 https://doi.org/10.1021/acs.molpharmaceut.0c01053
Zahmanova G, Mazalovska M, Takova K, Toneva V, Minkov I, Peyret H, Lomonossoff G (2021) Efficient Production of Chimeric Hepatitis B Virus-Like Particles Bearing an Epitope of Hepatitis E Virus Capsid by Transient Expression in Nicotiana benthamiana. Life (Basel, Switzerland) 11(1) doi: 10.3390/life11010064
Ponndorf D, Meshcheriakova Y, Thuenemann EC, Dobon Alonso A, Overman R, Holton N, Dowall S, Kennedy E, Stocks M, Lomonossoff GP, Peyret H (2021) Plant-made dengue virus-like particles produced by co-expression of structural and non-structural proteins induce a humoral immune response in mice. Plant Biotechnology Journal. doi: 10.1111/pbi.13501
Berardi A, Baldelli Bombelli F, Thuenemann EC, Lomonossoff GP, (2019). Viral nanoparticles can elude protein barriers: exploiting rather than imitating nature. Nanoscale. 11(5):2306-2316. doi: 10.1039/c8nr09067j.
Byrne MJ, Steele JFC, Hesketh EL, Walden M, Thompson R, Lomonossoff GP & Ranson NA. (2019) Combining transient expression and cryo-EM to obtain high resolution structures of luteovirid particles. Structure. https://doi.org/10.1016/j.str.2019.09.010
Hesketh EL, Tiede C, Adamson H, Adams TL, Byrne MJ, Meshcheriakova Y, Kruse I, McPherson MJ, Lomonossoff GP, Tomlinson DC, Ranson NA. (2019) Affimer reagents as tools in diagnosing plant virus diseases. Sci Rep. 2019 May 17;9(1):7524. doi: 10.1038/s41598-019-43945-6. PMID: 31101847
Peyret H, Brown J, Lomonossoff G (2019) Improving plant transient expression through the rational design of synthetic 5’ and 3’ untranslated regions. Plant Methods 15: 108 http://dx.doi.org/10.1186/s13007-019-0494-9
Stephenson MJ, Reed J, Brouwer B, Osbourn A (2018). Transient Expression in Nicotiana Benthamiana Leaves for Triterpene Production at a Preparative Scale. J Vis Exp. (138). doi: 10.3791/58169.
Castells-Graells R, Lomonossoff GP, Saunders K (2018) Production of Mosaic Turnip Crinkle Virus-Like Particles Derived by Coinfiltration of Wild-Type and Modified Forms of Virus Coat Protein in Plants. Methods Mol Biol. 1776:3-17. doi: 10.1007/978-1-4939-7808-3_1.
Thuenemann EC, Lomonossoff GP (2018). Delivering Cargo: Plant-Based Production of Bluetongue Virus Core-Like and Virus-Like Particles Containing Fluorescent Proteins. Methods Mol Biol. 1776:319-334. doi: 10.1007/978-1-4939-7808-3_22.
Wege C, Lomonossoff GP (2018). Virus-Derived Nanoparticles for Advanced Technologies. Methods and Protocols Humana press 1776 671
Brillault L, Jutras PV, Dashti N, Thuenemann EC, Morgan G, Lomonossoff GP, Landsberg MJ, Sainsbury F (2017). Engineering Recombinant Virus-like Nanoparticles from Plants for Cellular Delivery. ACS Nano. 11(4):3476-3484. doi: 10.1021/acsnano.6b07747.
Mardanova ES, Blokhina EA, Tsybalova LM, Peyret H, Lomonossoff GP, Ravin NV. (2017). Efficient Transient Expression of Recombinant Proteins in Plants by the Novel pEff Vector Based on the Genome of Potato Virus X. Front Plant Sci. 8:247. doi: 10.3389/fpls.2017.00247.
Meshcheriakova Y, Durrant A, Hesketh EL, Ranson NA, Lomonossoff GP, (2017). Combining high-resolution cryo-electron microscopy and mutagenesis to develop cowpea mosaic virus for bionanotechnology. Biochem Soc Trans. 45(6):1263-1269. doi: 10.1042/BST20160312.
Saunders K, Lomonossoff GP, (2017). In Planta Synthesis of Designer-Length Tobacco Mosaic Virus-Based Nano-Rods That Can Be Used to Fabricate Nano-Wires. Front Plant Sci. 8:1335. doi: 10.3389/fpls.2017.01335.
Steele JFC, Peyret H, Saunders K, Castells-Graells R, Marsian J, Meshcheriakova Y, Lomonossoff GP. (2017). Synthetic plant virology for nanobiotechnology and nanomedicine. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 9(4). doi: 10.1002/wnan.1447.
Lebedev N, Griva I, Dressick WJ, Phelps J, Johnson JE, Meshcheriakova Y, Lomonossoff GP, Soto CM. (2016). A virus-based nanoplasmonic structure as a surface-enhanced Raman biosensor. Biosens Bioelectron. 77:306-14. doi: 10.1016/j.bios.2015.09.032.
Lomonossoff GP and D’Aoust MA (2016). Plant-produced biopharmaceuticals: A case of technical developments driving clinical deployment. Science 353(6305):1237-40. doi: 10.1126/science.aaf6638.
Saxena P, Thuenemann EC, Sainsbury F, Lomonossoff GP (2016). Virus-Derived Vectors for the Expression of Multiple Proteins Plants. Methods Mol Biol. 1385:39-54. doi: 10.1007/978-1-4939-3289-4_3.
Peyret H., Lomonossoff G. P. (2015). When plant virology met Agrobacterium: the rise of the deconstructed clones. Plant Biotechnol J. 13(8):1121-35. doi: 10.1111/pbi.12412.
Saunders K, Lomonossoff GP. (2015). The Generation of Turnip Crinkle Virus-Like Particles in Plants by the Transient Expression of Wild-Type and Modified Forms of Its Coat Protein. Front Plant Sci. 6:1138. doi: 10.3389/fpls.2015.01138.
Meshcheriakova YA, Saxena P, Lomonossoff GP (2014). Fine-tuning levels of heterologous gene expression in plants by orthogonal variation of the untranslated regions of a nonreplicating transient expression system. Plant Biotechnol J. 12(6):718-27. doi: 10.1111/pbi.12175.
Sainsbury F, and Lomonossoff GP, (2014). Transient expressions of synthetic biology in plants. Curr Opin Plant Biol. 19:1-7. doi: 10.1016/j.pbi.2014.02.003.