Carbohydrate engineering
Workpackage G: Carbohydrate engineering
Plants provide unrivalled opportunities for provision of sugars and polysaccharides for biorefining, biofuels, animal feed, food and other industrial uses. The main goal of this workpackage is to improve the quality and increase the yield of target polymers, and to alter their structure and hence properties for higher value applications. The targets will be plant cell wall polymers, and storage carbohydrates of plants and algae. The objectives will be achieved by (i) building a registry of polysaccharide synthesis pathway genes and transcription factors that can be co-ordinately expressed using tested promoters from this and other workpackages, and (ii) genome engineering to generate novel carbohydrates with improved nutritional performance, providing an example of application of synthetic biology for potential societal benefit.
OpenPlant is developing a library of DNA parts for engineering of carbohydrates in plants.
A toolkit of algal glucan-active enzymes
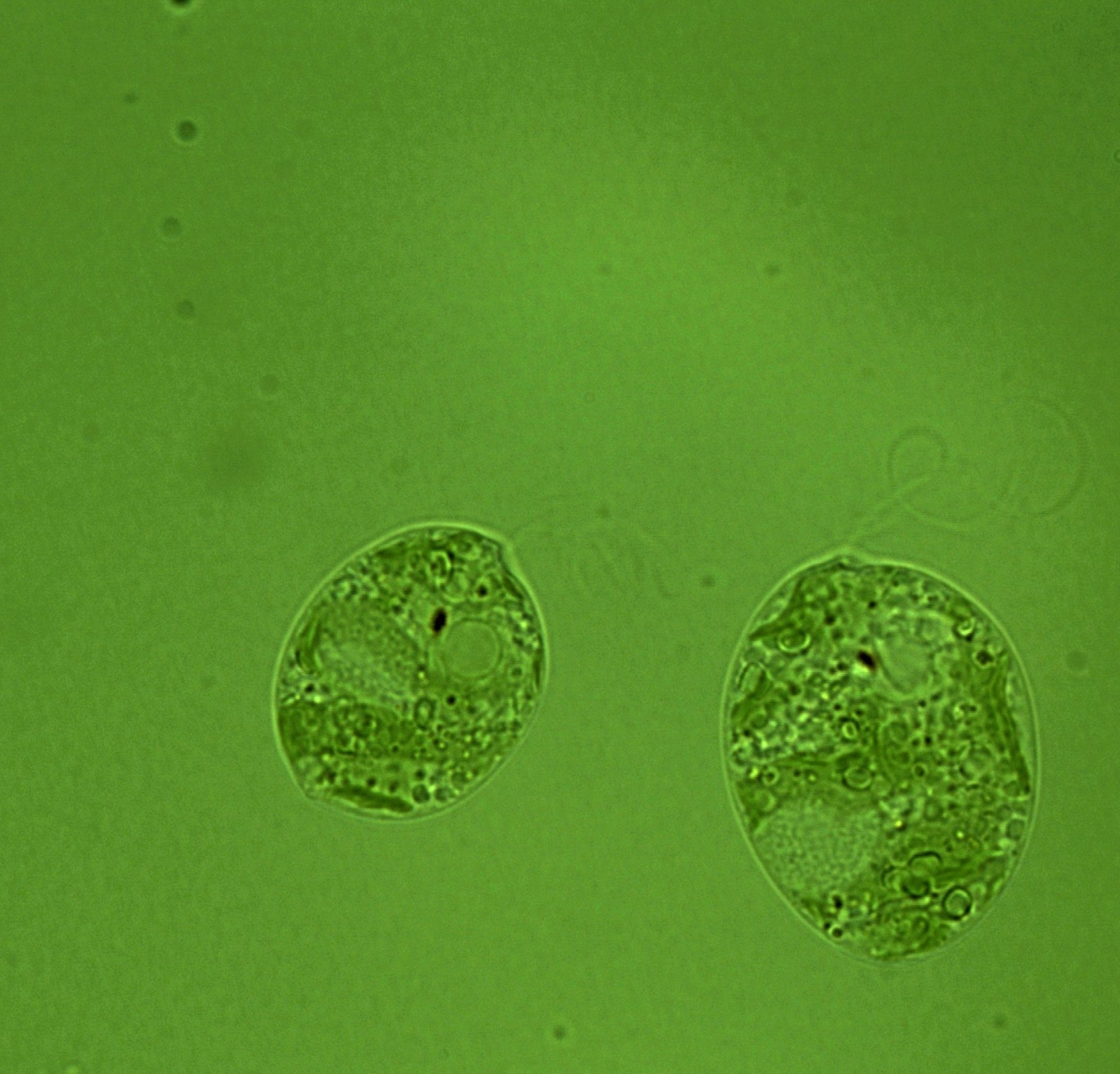
A complete informatics analysis has been conducted on two transcriptome datasets generated by the Field lab for the photosynthetic protozoan Euglena gracilis, cultured under autotrophic and heterotrophic conditions (O’Neill et al., 2015). This identifies an unexpectedly large repertoire of carbohydrate active enzymes, including many involved in storage, beta-glucan metabolism and a range of what appear to be hemicellulose synthesizing enzymes, although Euglena is not known to produce such glycans. The above mentioned analysis of the Euglena transcriptome has been released in the Carbohydrate-Active Enzymes (CAZy) database, with primary data in the process of being deposited through EBI. All data is available via the JIC web site: http://jicbio.nbi.ac.uk/euglena/
Image: Euglenia, shared at JIC bio
Advanced bioinformatics and structure homology prediction approaches are being used to identify candidate algal beta-1,3 glucan phosphorylases.
Further analyses of algae, such as Emiliania and Prymnesium, is ongoing together with an Innovate UK funded project, to assess their repertoire of polysaccharide and natural product glycosylation capabilities to feed into synthetic biology and industrial biotechnology studies.
Engineering carbohydrate content in potatoes
Aytug Tuncel (Smith lab, JIC) has applied and tested the genome editing tools and technologies developed in the Patron lab to generate novel, commercially or nutritionally valuable glucans in model plant and crop species. The primary objective is the creation of potatoes that contain digestion-resistant starches with two major nutritional benefits: reduced calorie intake from consumption of chips, crisps and other potato-based snack foods, and increased supply of complex carbohydrates to the microbiota of the lower gut that reduces risk of several diseases including colorectal cancer and type II diabetes. In collaboration with the BRACT group, constructs encoding RNA-guided Cas9 targetting starch-branching enzymes in the potato genome have been assembled and delivered to potato explants and potato protoplasts, giving rise to potato plantlets edited to different degrees in genes encoding two isoforms of starch-branching enzymes. Starch from mature tubers of these new genotypes have been tested in conjunction with experts on starch structure and digestion at the Institute of Food Research, Norwich. Read more about this work in Tuncel et al. (2019).
Engineering mannan and xylan in fibre cells
Section through Gnetum wood, confocal image: Jim Haseloff
The Dupree lab is designing inducible expression systems in fibre cells to engineer mannan and xylan synthesis. After cellulose, the hemicelluloses xylan and mannan are the most abundant polysaccharides on the planet. This project aims to modify the synthesis of these cell wall components by modifying their synthesis in the Golgi apparatus. Using specific promoters driving effective and specific glycosyltransferase (GT) activities is the cornerstone to achieve it, therefore the Dupree group has prepared constructs for expressing specific GT activities using a panel of validated tissue-specific promoters that are specific to secondary cell wall synthesising cells. Constructs for plant transformation were made with the OpenPlant Goldengate system. The constructs have been used to test promoter and GT combinations for hemicellulose modification, which has resulted in successful examples for re-engineering of cell wall polysaccharide synthesis. Read more about this work in Temple et al. (2019), Yu et al. (2018), and Lyczakowski et al. (2017).
Publications
Dedola S, Rugen MD, Young RJ, Field RA (2020) Revisiting the Language of Glycoscience: Readers, Writers and Erasers in Carbohydrate Biochemistry. ChemBioChem 2020, 21, 423.
Kuhaudomlarp S, Pergolizzi G, Patron NJ, Henrissat B, Field RA (2019) Unraveling the subtleties of β-(1→3)-glucan phosphorylase specificity in the GH94, GH149 and GH161 glycoside hydrolase families. J Biol Chem. 2019 Apr 19;294(16):6483-6493. doi: 10.1074/jbc.RA119.007712.
Kuhaudomlarp S, Walpole S, Stevenson CEM, Nepogodiev SA, Lawson DM, Angulo J, Field RA (2019). Unravelling the Specificity of Laminaribiose Phosphorylase from Paenibacillus sp. YM-1 towards Donor Substrates Glucose/Mannose 1-Phosphate by Using X-ray Crystallography and Saturation Transfer Difference NMR Spectroscopy. Chembiochem. 20(2):181-192. doi: 10.1002/cbic.201800260.
Temple H, Mortimer JC, Tryfona T, Yu X, Lopez‐Hernandez F, Sorieul M, Anders N, Dupree P (2019) Two members of the DUF579 family are responsible for arabinogalactan methylation in Arabidopsis. Plant Direct. 3(2):e00117. doi: 10.1002/pld3.117.
Wagstaff BA , Rejzek M , Kuhaudomlarp S, Hill L, Mascia I, Nepogodiev SA, Field RA (2019) Discovery of an RmlC/D fusion protein in the microalga Prymnesium parvum and its implications for NDP-β-l-rhamnose biosynthesis in microalgae. J Biol Chem. 294(23):9172-9185. doi: 10.1074/jbc.RA118.006440.
Tuncel A, Corbin KR, Ahn‐Jarvis J, Harris S, Hawkins E, Smedley MA, Harwood W, Warren FJ, Patron NJ, Smith AM (2019) Cas9-mediated mutagenesis of potato starch branching enzymes generates a range of tuber starch phenotypes. Plant Biotechnol J. 17(12):2259-2271. doi: 10.1111/pbi.13137.
Kuhaudomlarp S, Patron NJ, Henrissat B, Rejzek M, Saalbach G, Field RA (2018). Identification of Euglena gracilis β-1,3-glucan phosphorylase and establishment of a new glycoside hydrolase (GH) family GH149. J Biol Chem. 293(8):2865-2876. doi: 10.1074/jbc.RA117.000936.
Wangpaiboon K, Padungros P, Nakapong S, Charoenwongpaiboon T, Rejzek M, Field RA, Pichyangkura R, (2018). An a-1,6-and a-1,3-linked glucan produced by Leuconostoc citreum ABK-1 alternansucrase with nanoparticle and film-forming properties. Sci Rep. 8(1):8340. doi: 10.1038/s41598-018-26721-w.
Yu L, Lyczakowski JJ, Pereira CS, Kotake T, Yu X, Li A, Mogelsvang S, Skaf MS, and Dupree P. (2018) The Patterned Structure of Galactoglucomannan Suggests It May Bind to Cellulose in Seed Mucilage. Plant Physiol. 178(3): 1011–1026. doi: 10.1104/pp.18.00709
Lyczakowski JJ, Wicher KB, Terrett OM, Faria-Blanc N, Yu X, Brown D, Krogh KBRM, Dupree P, Busse-Wicher M. (2017). Removal of glucuronic acid from xylan is a strategy to improve the conversion of plant biomass to sugars for bioenergy. Biotechnol Biofuels.10:224. doi: 10.1186/s13068-017-0902-1.
O’Neill EC, Kuhaudomlarp S, Rejzek M, Fangel JU, Alagesan K, Kolarich D, Willats WGT, Field RA (2017). Exploring the glycans of Euglena gracilis. Biology (Basel) 6(4). pii: E45. doi: 10.3390/biology6040045.
O'Neill EC, Pergolizzi G, Stevenson CEM, Lawson DM, Nepogodiev SA, Field RA, (2017). Cellodextrin phosphorylase from Ruminiclostridium thermocellum: X-ray crystal structure and substrate specificity analysis. Carbohydr Res. 451:118-132. doi: 10.1016/j.carres.2017.07.005.
Rejzek M, Hill L, Hems ES, Kuhaudomlarp S, Wagstaff BA, Field RA (2017). Profiling of Sugar Nucleotides. Methods Enzymol. 597:209-238. doi: 10.1016/bs.mie.2017.06.005.
O’Neill EC, Saalbach G, Field RA. (2016). Gene Discovery for Synthetic Biology: Exploring the Novel Natural Product Biosynthetic Capacity of Eukaryotic Microalgae. Methods Enzymol. 576:99-120. doi: 10.1016/bs.mie.2016.03.005.
O'Neill EC, Trick M, Hill L, Rejzek M, Dusi RG, Hamilton CJ, Zimba PV, Henrissat B, Field RA. (2015). The transcriptome of Euglena gracilis reveals unexpected metabolic capabilities for carbohydrate and natural product biochemistry. Mol Biosyst. 11(10):2808-20. doi: 10.1039/c5mb00319a.